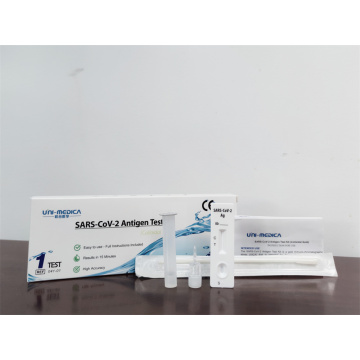
SARS-CoV-2 and Omicron variant Antigen Rapid Test Kit
| Min. Order: | 10000 test |
|---|
| Packaging: | export standard |
|---|---|
| Productivity: | 100,000,000 pcs/year |
| Brand: | Uni-medica |
| Transportation: | Ocean,Land,Air,Express |
| Place of Origin: | China |
| Supply Ability: | 10,000,000,000,000 pcs/year |
| Certificate: | CE/SGS |
| HS Code: | 38220090 |
| Port: | Shenzhen,Guangzhou,Hongkong |
Basic Info
Model No.: R041-01
Click on the follow link to find out more information: https://www.unimed-global.com/antigen-test-kit-of-covid-19/
Company Info
- Company Name: Shenzhen Uni-medica Technology Co.,Ltd
- Representative: Wang yan Pin
- Product/Service: Total solution of SARS-CoV-2 , RT-PCR test kits , Multiplex Fluorescence Staining Solution , Pathogens targeted NGS , Newborn Genetic Screening NGS , Immunochromatography POCT
- Capital (Million US $): 5,000,000RMB
- Year Established: 2011
- Total Annual Sales Volume (Million US $): US$10 Million - US$50 Million
- Export Percentage: 51% - 60%
- Total Annual Purchase Volume (Million US $): US$5 Million - US$10 Million
- No. of Production Lines: 5
- No. of R&D Staff: 71 -80 People
- No. of QC Staff: 51 -60 People
- OEM Services Provided: yes
- Factory Size (Sq.meters): 1,000-3,000 square meters
- Factory Location: 2nd Floor, No.6 Building, Xili Liuxian Culture Park, Nanshan District, Shenzhen City, Guangdong Province, China
- Contact Person: Ms. Super D~D
- Tel: +86-755-86505501
Premium Related Products
Other Products
Hot Products
Thermal Warm GloveNitrile Garden GloveCut Resistant GloveTop Grain Cowhide Safety Working GlovesNitrile Working GloveHeat Resistant Cow Split Leather Welding Apron for WeldersHeat Resistant Baking and Oven GlovesAnti Slip, Nitrile Coated GloveFurniture Leather Working Safety Protective Hand Gloves for RiggersAb Grade Cow Split Leather Safety Working Drivers Gloves for Driving13 Gauge Polyester Liner, Polyurethane Coating GloveGoat Grain Leather Drivers Driving GlovesPig Skin Safety Working Gloves for RiggersCow Grain Leather Working Driving Gloves for Drivers13 Gauge Nylon Liner, Latex Coating, Crinkle Finish Glove13 Gauge Polyester Nitrile Working Glove